Haim Pharma’s Solution: Psilocin-Based Therapeutics for Neurodegenerative Diseases
Psilocin, the active compound derived from psilocybin, exhibits multimodal activity that shows promising therapeutic potential for neurodegenerative diseases. By addressing key disease pathways, psilocin has the potential to slow disease progression in conditions such as Parkinson’s Disease (PD) and Amyotrophic Lateral Sclerosis (ALS), among others.
Unique Product Approach
Our approach to psilocin-based therapeutics prioritizes a targeted, patient-centered design. Each step of our product development focuses on maximizing treatment effectiveness while minimizing side effects, setting us apart from traditional approaches:
Patient-Centric Development
We put patients at the center of our design process, focusing on targeted treatments that minimize side effects, thereby improving patients’ quality of life.
Scientific Rigor and Evidence-Based Development
Our product development is deeply rooted in scientific evidence, incorporating rigorous testing and data-driven strategies to establish the most effective dosages and treatment regimens for specific neurodegenerative conditions.
Mechanism-Specific Dosing Strategies
We are committed to identifying optimal dosage levels aligned with specific mechanisms of action, ensuring that each formulation is efficacy-driven and supports patients’ therapeutic needs.
Cost-Effective Virtual Drug Development
By adopting a virtual drug development model, we significantly reduce costs while maintaining high standards of quality and adaptability, ultimately providing a more affordable therapeutic option.
Collaborative Innovation and Rapid Access to Expertise
Through strategic collaborations with leading researchers and experts, we gain fast access to the latest insights and innovations, allowing us to bring advanced, flexible treatments to market faster.
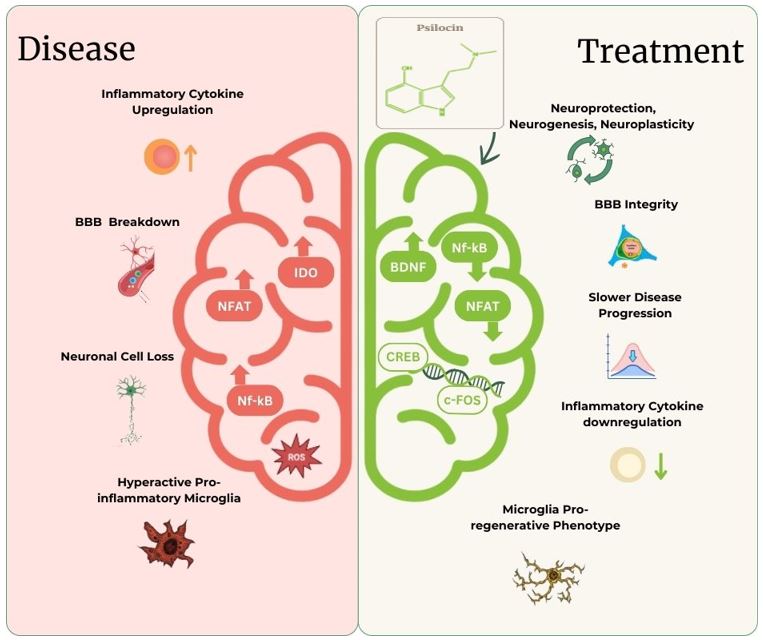
Our Vision: Safe, Effective, and Accessible Psilocin-Based Treatments
Our mission is to introduce a psilocin-based therapeutic solution that is safe, effective, and accessible, tailored to tackle the complex symptoms of neurodegenerative diseases. We are committed to developing treatments that improve patients’ quality of life and target disease progression, providing hope for those living with challenging neurological conditions.
Commitment to Regulatory Standards
Founded in April 2022, Haim Pharma Group (HPG) was established with a clear mission to develop scientifically driven treatments for neurodegenerative diseases. We are committed to regulatory excellence and aim to achieve FDA and EMA approval for each of our products. Through systematic adherence to regulatory protocols, we ensure our solutions meet the highest standards of safety and efficacy, reinforcing our dedication to advancing patient health.
